Producing Light
How can light be produced?
Obviously one way is using lasers - something that we will study in detail in this course later on. Here we want to take up other ways in which light can be produced including natural and man-made sources.
Natural sources of light include our sun and other stars, where the source of energy is nuclear energy (recall that the moon does not produce light but merely reflects sunlight), lightning, where the source is electrical, and fire, where the energy source is chemical. For eons of human history, these were the only sources of light available until people discovered burning natural oils and developed candles in the relatively recent past.
Artificial lightning generated by static electricity in a van de Graff machine
Man-made modern sources of light are mainly of three types: incandescent and fluorescent light bulbs and discharge tubes, such as the well-known neon tubes used in store signs. The simplest of these devices to understand is the incandescent light bulb. In order to understand how the other two work we will first need to learn a good deal about the atomic structure of matter.
A light bulb consists of the glass bulb enclosing a tungsten wire filament that makes electrical connection with the universal metal base that screws into an electrical outlet. The bulb is at reduced atmospheric pressure (about 80% of atmospheric) usually containing an inert gas such as nitrogen. Tungsten is used for the filament first because it is a metal and will conduct electricity, and second because it has a high melting point (6,580 oF). When the light bulb is "turned on," meaning that an electric current, or stream of electrons, passes through the tungsten wire, the wire heats up from collisions of the electrons with the tungsten atoms. The electrons are being steadily pushed by the external energy from the electrical outlet and transfer energy to the tungsten atoms through collisions.
(left) A variety of light bulbs with the filament visible in the clear one; (right) A clear light bulb with light being emitted from the hot filament
Hot objects glow and give off light, just as the coils of a toaster glow red, or the wood and coals of a fire give off light. The colors of a flame actually indicate the local temperature of the gases. At temperatures above 1000 oF objects will glow and give off visible light that we call incandescent. This thermal radiation has only a small portion in the visible (about 5% for a fire) while most of the rest is infrared radiation. You can feel infrared radiation as the heat from a fire. Thermal radiation is the name for electromagnetic radiation emitted by the huge numbers of electrons in hot objects and as such, if the wavelengths of the light are examined, there is a continuous distribution of all wavelengths or frequencies present. The hotter the object, the more energetic or higher is the average frequency of the light, but the light consists of a broad continuous distribution of frequencies spanning a wide range of the electromagnetic spectrum.
To learn how a fluorescent light or a discharge tube work, we need first to understand some more about the atomic structure of matter.
There are three separate types of constituents, or elementary particles, that make up a typical atom: electrons, protons, and neutrons.
Protons and neutrons form a very tightly bound structure within the atom, called the atomic nucleus. Electrons are, in turn, bound to this nucleus, but not as tightly or as strongly as are the nuclear components to one another.
In terms of their properties, protons and neutrons are almost identical. The major difference between these two is that protons have positive electric charge, while neutrons have no electric charge - they are neutral, as suggested by their name. Electrons are also charged. In fact, their charge is exactly the same magnitude (amount) as that of the proton, but negative in value. Another measurable property of these constituents is their mass. Protons and neutrons have very nearly the same mass; each is about 2,000 times more massive than an electron. So, for the most part, it is the nucleus of the atom that determines the mass of the atom, and not the electrons that surround it. But the size of the atom is determined by the spread of the electrons around the nucleus and a typical atom is about 100,000 times the size of its nucleus. If we imagine that we zoom-in on an atom so that its nucleus gets to be about one foot in diameter, then the electrons of the atom spend most of their time at distances of about 100,000 feet from this nucleus. Said another way, if we zoom-in on an atom so that it is the size of a football field (about 100 meters) then the nucleus will be the size of the head of a pin (about 1 mm).
These constituents - protons, neutrons, and electrons - are not necessarily fixed entities, like objects that we deal with in our every day lives. For example, to-date, we have not measured a finite size for the electron, despite the fact that in many instances electrons behave as if they are particle like material objects. The electron appears smaller than we have been able to measure. Still, these constituents have identifiable (measurable) properties that allow us to classify them. Also, even though we refer to the proton and neutron as "elementary", they in turn are made of other (and therefore more elementary) particles, called quarks. Further, because they are made from quarks, protons and neutrons can often turn into other particles; for example, the neutron can spontaneously turn into a proton plus an electron plus a third neutral fundamental particle, called a neutrino - from the Italian for little neutron.
Questions on Atomic Constituents
There are three types of fundamental forces in nature. These are ranked according to their increasing strength, gravitational, electro-weak, and hadronic. In our current and most widely accepted model of particle physics, known as the Standard Model, these forces are all that one needs in order to explain all physical interactions in nature.
Each of these forces has its own signature, i.e. behavior. The force of gravity, for instance, is always attractive. For two objects to interact through this force they each need to have mass. So, any object that has mass attracts all other objects that have mass. The strength of this attraction depends on the amounts of each object's mass as well as on the distance separating the objects. The larger the masses, the stronger the force; and the closer the masses, the stronger the force. The exact mathematical relationship that describes this force was discovered, over 300 years ago, by Isaac Newton and is called Newton's Law of Gravitation. The most familiar form of this force is our weight, due to the attraction of our mass to that of the earth.
The electro-weak force exhibits three separate forms of interaction: electric, magnetic, and a so- called "weak" form. The electric and magnetic forces together were fully understood through a set of 4 equations by Maxwell, about 150 years ago and is often called the electromagnetic force. The required property that two objects must have in order to interact via the electromagnetic force is not mass, but electric charge, which can be either positive or negative. Electric charge comes in a smallest unit - namely the charge on the electron or proton - and is said to be quantized. The total electric charge on an object must therefore be in multiples of this fundamental unit of charge. Unlike gravity which is always attractive, this interaction can be either attractive, when the objects have charges of opposite sign (+ & -), or repulsive, when they have charges of the same sign (+ & +, or - & -). A second important difference is that this force is much stronger than the gravitational force. For example, if we consider two protons, their force of gravitational attraction is so much weaker than their electric repulsion that, for all practical purposes, we can ignore the gravitational effects for them altogether. In fact, were it not for the fact that atoms, by virtue of having an equal number of electrons and protons, are neutral we would not have to even bother with the gravitational force! Interestingly, for two stationary charges, the form of this force is very similar to that for the gravitational force between two masses. The magnetic interaction takes hold when electric charges move to produce electric currents. It is the electric current that produces the magnetic force. All other forces than gravity that we directly experience are electromagnetic in origin - all of chemistry (except nuclear chemistry), pushes, pulls, tension forces, motor forces, etc., are all electromagnetic. The weak force is responsible for radioactivity and is one of two nuclear forces that only act inside the nucleus. The theory for the electro-weak force was developed by Glashow, Weinberg and Salam, for which they received the Nobel prize in 1979.
The hadronic force acts between protons and neutrons in the same exact way. This force tends to be fully repulsive at extremely small separation distances, then very attractive as these particles just begin to separate. But, unlike either gravitational or electric forces, it rapidly loses its influence with larger separation distances outside the nucleus, just like the weak force. For these reasons, this force is referred to as one with a "hard core" and a "short range". In contrast, both the gravitational and electromagnetic forces are "long range".
A basic philosophical/scientific question arises for long range forces - namely, when two particles are separated in space, how does the information that they exert forces on each other travel between these particles? Our current response would be that, depending on the type of interaction, there are mediating "virtual" particles that travel at the speed of light back and forth between the two interacting particles, constituting a field. For example, in the case of electromagnetic interactions, these mediating particles are "virtual" photons while in the case of hadronic forces they are known as gluons (a suggestive term indicating that they hold the protons together that would otherwise want to fly apart due to their electrical repulsion). Physicists anticipate that the three forces of nature will eventually be united in a single "theory of everything." The current best candidate for this uses mathematical objects known as "strings" - in string theory.
Energy is an important physical concept that is a measure of the strength of an interaction within a process. We can distinguish two classes of energy for an object: energy due to the motion of the object, known as kinetic energy, and energy due to interactions with the outside world, known as potential energy. Kinetic energy for a particle of mass m is given by the equation KE = ½mv2 so the more massive and faster a particle travels, the greater is its kinetic energy. Potential energy has many different forms, each arising from the specific type of interaction, such as gravitational or electromagnetic. In the case of an atom, the interaction of electrons with the atomic nucleus, via the electromagnetic force, and the interaction of the nuclear constituents, via the hadronic force, are responsible for its potential energy. Any process that alters the force of interaction between an electron and the nucleus, say by increasing their separation distance, changes the atom's energy. In this way atoms can gain or lose energy through interactions with their surroundings.
One of the most important principles of science is Conservation of Energy. It is very much like a bookkeeping device that is universally true. It says that in an isolated system (one which does not exchange matter or energy with its external environment) if you total up the energy of everything in the system at one time, that energy will always remain constant. All forms of energy must be included in this tally and the system must remain isolated over time. This principle is the basis for much of our future discussions, and will often be invoked without explicitly saying so.
Questions on Atomic Forces and Energies
Atoms are normally neutral. They each have equal number of protons and electrons. But despite this fact, the primary force of interaction between two atoms is electric. This is because electrons in a given atom are so far (relative to the nuclear size) away from their own atomic nucleus that electrons from two separate atoms can not only interact with each other (repulsion), but also interact with the nucleus of the other atom (attraction).
When an atom is missing one or more electrons, it is, in effect, positively charged. But its mass remains roughly the same as when it had all of its electrons since the nucleus contains more than 99.9% of the atom's mass. In contrast, if it were to be missing one or more protons, its mass would become substantially different. So, what determines the species of the atom is the proton number. Different atomic elements are therefore given different names because they have different proton numbers. Of the stable atoms, hydrogen has the lowest proton number of 1. Next, is helium with 2 protons, then lithium with 3, etc. These and all other known atoms are listed in the periodic table of elements. See the table of atomic elements on the NIST server.
Atoms of a given species can not only lose one or all of their electrons, but they can also gain extra electrons. By losing electrons atoms become positively charged and by gaining additional electrons they become negatively charged. Such effectively charged atoms are called ions.
Two atoms with same number of protons, but different number of neutrons still belong to the same species (same element), but to different isotopes. Some atoms have many stable isotopes and some only a few. Hydrogen has three, each with one proton: the regular (most abundant form) atom with no neutrons, deuterium with one neutron, and tritium with two neutrons. It is the nature of the hadronic force that determines the number of stable isotopes for each atom.
When two or more atoms bond together they form a molecule. The molecule as a whole behaves similarly to an atom, since it is made of the same constituents interacting with one another just as they do in an atom. In addition, a molecule exhibits other features that are explained by modeling it to be made of individual, intact, atoms, the atoms of the molecule acting as "separate" entities. One of these additional features is that the molecular (kinetic) energy of motion can be divided up among kinetic energies to make their atoms vibrate, rotate, as well as to simply translate the molecule. These new channels of energy do not exist for individual atoms in the same way. The structure as well as dynamics of molecules, therefore, can be far more complex.
Until the discovery of electrons and protons, both in the late 19th century, atoms were thought to be the most minute structure in nature. An atom was "modeled" by a point - the smallest imaginable point. But the discovery of atomic constituents required a more elaborate model than a point object model. It was initially proposed that atoms are made of positive charge centers scattered in a continuum of negative charge, similar to raisins in raisin bread. This model seemed to agree rather well with experimental evidence, but further experimentation required major modifications.
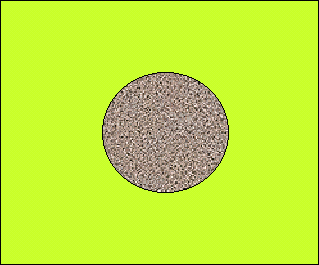
Raisin bread model of an atom with lumps of positive charge distributed throughout the negative charge
The most striking experimental evidence against such a model was that light emitted by well separated atoms (like atoms in a gas or vapor) was always missing some colors. In fact, in the case of most atoms only a handful of colors were visible. These colors, known as the atomic spectrum, were produced in the laboratory by burning atomic vapors or by passing an electric current through them. The consistency of the spectrum for a given atom clearly suggested that atoms emitted light of very distinct - signature - colors, unlike all other sources of light known. Further experimentation suggested that light itself appears to be made of separate, yet identical, constituents called photons. Furthermore, the energy of a photon is directly related to its color. The more bluish the light (the higher the frequency), the higher is the energy of the photons that make it up. This quantization of light, evident by its photon nature, along with atomic spectra then forced the notion of quantization of the atom.
Quantization of the atom lead to Bohr's model that places the nucleus as the "sun" and electrons as planets revolving about it in fixed orbits analogous to our solar system. The picture of this model is often drawn not as orbits in one flat plane, as is the case for the solar system, but in the atomic planetary model the orbits of electrons are three dimensional. This orbital model of the atom was in fact very effective at first, but it too was shown to be inadequate. Despite the very limited use of the planetary model, it is this picture that is most widely used for depiction of the atom!
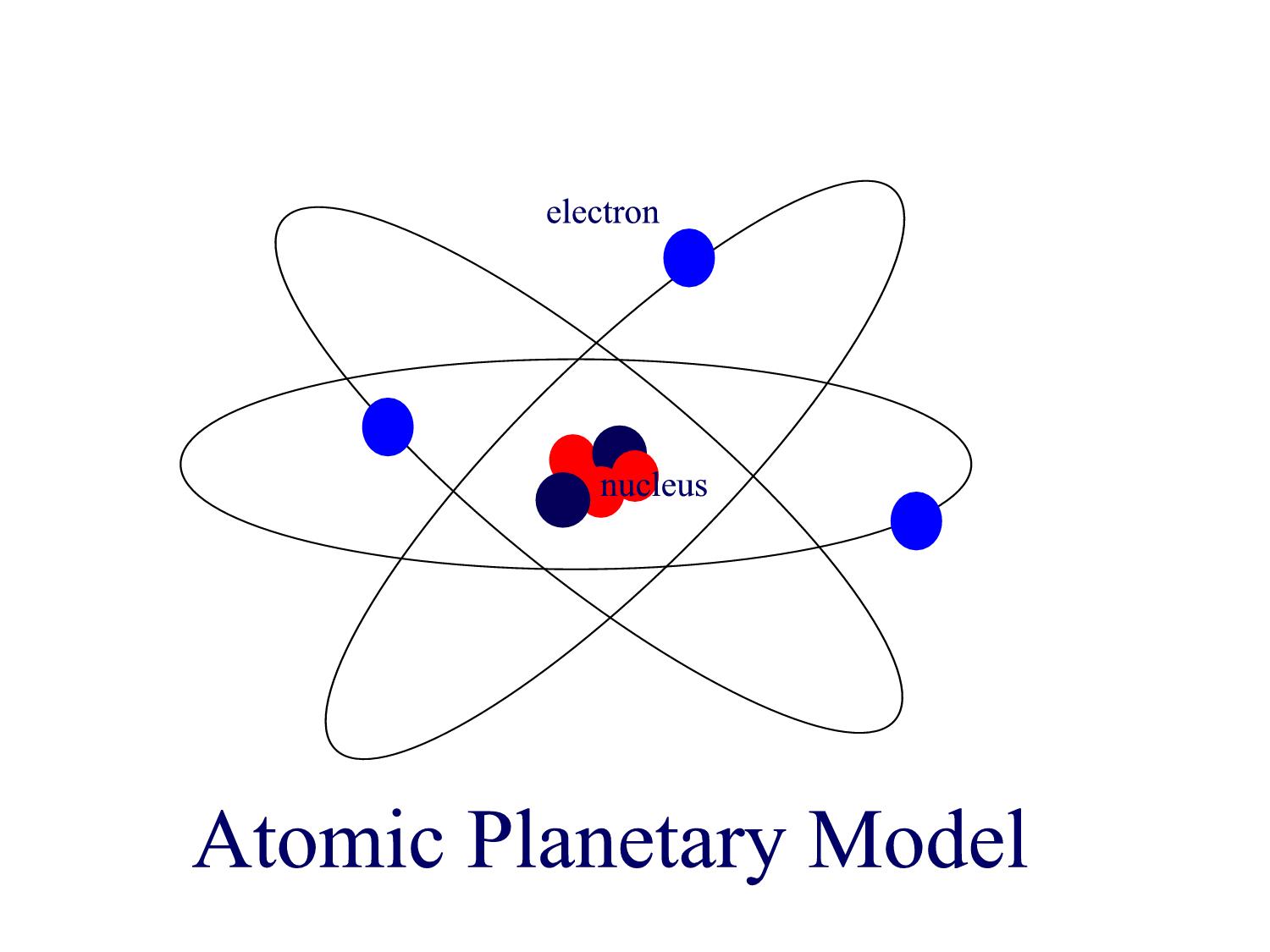
Strangely enough, the most successful model, and the one that agrees well with currently accepted theories of quantum mechanics, is a hybrid of these two models. In the electron cloud model the "presence" of the electron about the nucleus resembles the continuum of negative charge, the bread, in the raisin bread model. But unlike the raisin bread model, and just as in the planetary model, here all the positive charge is concentrated in the center of the atom in one single raisin, i.e. the nucleus.
The concept of quantization is not new to physics. In nature we are surrounded by quantized material of all sorts. Most living creatures (but not all) are quantized: they appear in separate units, as opposed to a continuum. Our neighbor may have one, two, three, ... dogs; but never 2.13577 dogs! Still, quantized properties are rare. Most objects that we encounter in our every day lives have properties whose values can change continuously. But we find that in the case of small things, such as an atom, this is no longer the case. Most of the properties of very small things turn out to come in an integer multiple of a "smallest" unit called a quanta. In particular, light of a given color is made of identical photons. Each of these has an energy Eg the value of which is a product of a constant, h, called Planck's constant, and the frequency of the photon, n.
Photon Energy: Eg = hn
where h has a value of 6.63x10-34 J.s and n is measured in cycles per second, i.e. Hz.
In this way the energy of light of a given color (wavelength/frequency) is quantized. There are other properties of very small (sub microscopic) entities that take just two values. One such property is the so called "spin". Photons (of any frequency) have a spin value that is equal to Planck's constant divided by 2p, no more and no less. Electrons and nucleons have half as much spin as do photons. This means that if two substances are to increase or decrease their net spin values by the exchange of electrons or nucleons, they can only do this in unit multiples of the spin of the electron or nucleon. This, of course, is very similar to currency exchanges that take place only in multiples of the smallest unit of currency.
Atomic quantization is a slightly different concept. Evidently, each atom is quantized based on that particular atom's makeup of electrons, protons, and neutrons and the interactions among these constituents. The (potential) energy of the atom, because of the dynamic nature of its constituents, can have different values. But these values are very much limited to very specific ones. Said differently, in the continuum of energy values that each atom could possibly have, the energy values that the atom can actually have are limited to a set characteristic of its species.
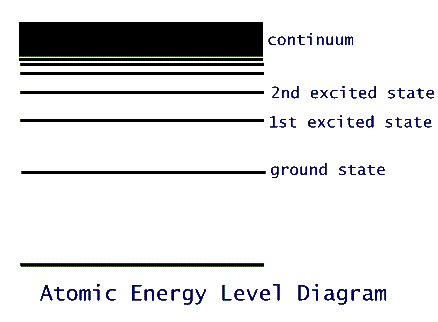
A stone of mass m is attracted to the surface of the earth because of the gravitational force of interaction between it and the earth. The (potential) energy of this earth-stone system depends on their distance of separation: U = mgH, where g is acceleration of gravity, 9.8 m/s2, and H is the height in meters that the stone is above the earth's surface. Because this distance, H, can vary continuously, the energy can take any value. If we draw a vertical line depicting this height, and therefore this energy, then the stone can "be" at any location on this line. In this analogy, an atom can only be at very specific points on this line, similar to a ladder arrangement. Each atomic species, then, has a ladder whose rungs are separated according a characteristic scale that fits that species. These rungs are referred to as the energy levels of the atom.
An energy ladder of sorts; at least you need some to climb it!
The lowest possible energy level of the atom is called its ground state. In nature, evidently, all things tend to seek to their lowest possible energy situation. So, any atom tends to remain in its ground state, unless it is forced out of it. All the other rungs, then, are called excited states. The one just above the ground state is called the first excited state; the one above it, the second excited state; and so on. In all atoms the rungs get closer and closer to each other the higher up this ladder we look. At the very top the rungs get so close to each other that for all practical purposes the energy scale gets to become a continuum.
An anonymous poem:
In the beginning ...
there was Aristotle,
And objects at rest tended to remain at rest,
And objects in motion tended to come to rest,
And soon everything was at rest
And God saw that it was boring.
Then God created Newton,
And object at rest tended to remain at rest,
And objects in motion tended to remain in motion,
And energy was conserved and momentum was conserved and matter was conserved
And God saw that it was conservative.
The God created Einstein,
And everything was relative,
And fast things became short,
And straight things became curved,
And the universe was filled with inertial frames
And God saw that it was relatively general, but some was especially relative.
Then God created Bohr,
And there was principle
And the principle was quantum,
And all things were still relative
And God saw that it was confusing.
And God was going to create Fergeson,
And Fergeson would have fielded a theory,
And he would have unified,
And all would have been one,
But it was the seventh day,
And God rested,
And objects at rest tend to remain at rest.
When atoms change their energy, by moving from one energy level to another, they are said to undergo a transition.
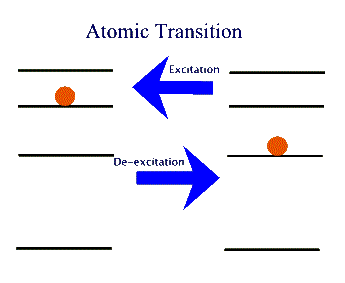
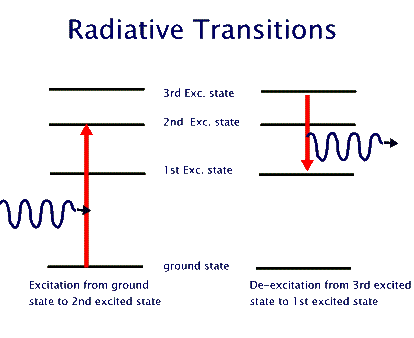
A transition in which the atom gains energy is called excitational; one in which it loses energy is then called de-excitational (see the above figure). There are two separate paths for either of these: Collisional and Radiative.
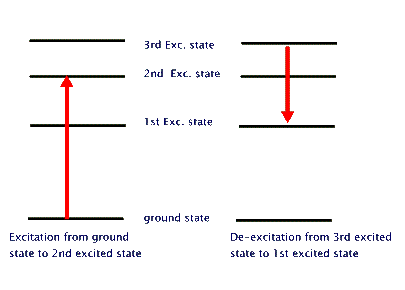
In a Collisional excitation the atom interacts, mostly, via electromagnetic interaction with another atom, an ion, or any other charged object and gains energy - as allowed by its energy level structure. The same process can alternatively take place in a Radiative transition, but this specifically requires the participation of a photon. That is to say, the atom absorbs one or more photons to "jump" from a lower energy level into a higher one. Similarly, in a de-excitation the atom jumps from a higher energy level to a lower one. If it gives this extra energy to another atom, ion, or charged object, then it has undergone a Collisional de-excitation. But if it just emits a photon with the extra energy, then it has undergone a Radiative de-excitation, or Radiative emission.
If a collection of identical atoms are excited by a broad energy source so that all of the excited states become populated, radiative de-excitation from the different states will produce an emission spectrum of emitted photons. Different atoms will have different sets of possible emitted photon energies that are characteristic of that element. For example, when hydrogen is excited in this manner, the de-excitation transitions are shown below.
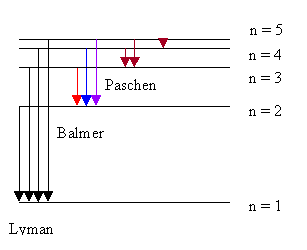

For hydrogen, it turns out that there are only three transitions that lead to photons of visible light (the three visible Balmer transitions shown above; a red, blue and violet color); the others are either infrared (dark red) or ultraviolet (black in the figure). The photo shows the hydrogen spectrum viewed through a diffraction grating that disperses the different colors of light. These spectra can, in fact, be used to identify the type of atoms that are emitting light. Measurement of the color composition of light from stars can give us information about the elements that are present in the stars.
Questions on Atomic Transitions
Now that we know something about atoms, we can discuss two other types of man-made light sources: discharge tubes and fluorescent light bulbs. Discharge tubes are glass tubes containing a gas at low pressure, so that the atoms in the tube are relatively far apart and do not interact strongly. A high voltage is applied across the tube between metal electrodes at either end. The flow of ions through the tube excites the gas atoms by collisional excitation. The radiative de-excitation produces light with specific colors, characteristic of the atom species present. Check out this web site for the spectra of all the elements. Sometimes the tubes are made of colored glass so that only one of the characteristic colors emerges. The most common example of the use of discharge tubes is in store signs using neon lights. A closely related second example is the fluorescent light bulb - specially designed to produce a type of white light.
(left) Two fluorescent bulbs, one clear and one frosted; (right) a variety of gas discharge tubes
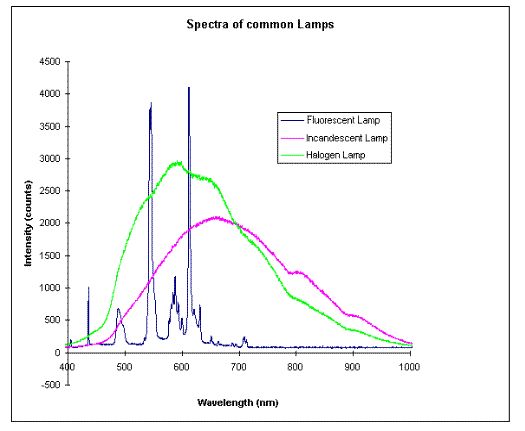
Fluorescent light bulbs contain a mixture of inert gases (usually argon and neon) together with a drop of mercury at low pressure, so that some of the mercury atoms form a gas in the tube. If you inspect a fluorescent light bulb you can sometimes see the characteristic mercury silver coating on parts of the glass near either end. Fluorescent light bulbs are plugged into special outlets that use a transformer (ballast) to produce a high voltage across the tube. The mercury vapor produces its characteristic discharge - just as in a mercury discharge tube - that has a strong ultraviolet component at around 250 nm wavelength. If this were all, the fluorescent light bulb would produce mainly uv light that we could not see. The inside of the glass tube is coated with a phosphor coating that is designed to absorb the uv light (radiative absorption) and re-emit a broad range of visible light with longer wavelengths (or lower frequencies or reduced energies). This process of absorbing light at shorter wavelengths (typically uv) with re-emission at longer wavelengths is called fluorescence. Depending on the phosphor coating, a different mix of visible colors blend to produce a light that appears to us white with different shadings. The following spectra show the distribution of light from three different sources. Note that incandescent and halogen lamps give continuous spectra, while fluorescent lamps give a mix of discrete bands of different wavelengths of light.
Questions on the Production of Light
