Electromagnetic Waves
Electrically charged objects attract or repel each other, just as two magnets attract or repel each other. The electric force that acts between charges has significant differences from the magnetic force that results in the interaction of magnets. But, for the topic at hand, these forces behave very much the same way in that they are both of the "action-at-a-distance" type. That is to say, these forces manifest themselves in the absence of any physical contact between the objects that interact with each other. For example, two magnets exert forces on each other even while they are apart and neither is touching the other. In fact, the reason that a compass works is that its small magnetized needle rotates because of the magnetic force of the earth, as if manipulated by a ghost. Another way (model) of explaining this interaction is to state that the earth's magnetic core establishes a magnetic field everywhere in space. It is this field that affects the compass needle. This concept of "field" is useful because once we know the field of the earth, then we know how any magnet would be affected once it enters this field. The field provides an intermediary to understand how action at a distance can work. With electric charges, each charge produces an electric field which then in turn interacts with other electric charges.
Another advantage of the field concept is that it allows for an easier visualization of what happens when one of the charges (or magnets) begins to move. In this view, when a charge changes position, the field that it produces also changes in space. In fact, as the charge oscillates, so does its field. This oscillating field is what is called an electromagnetic wave. By making a charge oscillate at one point in space we can cause another charge located further away to undergo oscillatory motion. Similar to mechanical waves, such as sound and water waves, electromagnetic waves are characterized by their frequency, speed, and amplitude.
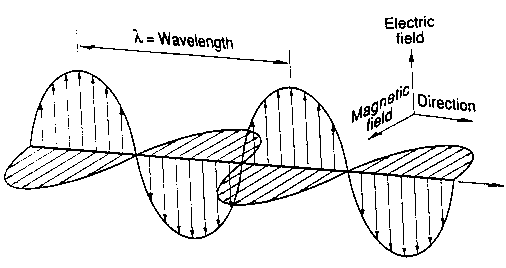
The above picture shows how both the magnetic and electric fields oscillate as the wave propagates to the right.
One interesting aspect of an electromagnetic wave that sets it apart from all other waves we have examined so far is that its propagation requires no medium. Water waves, which are transverse, of course need water to propagate in. Sound waves, which are longitudinal, also need a matter medium; although almost any type of matter would do for them (sound travels in air, all known gases, in fluids, in solids, and in plasma, a gas of charged ions). But oscillating electromagnetic fields travel even in vacuum. Another interesting feature of electromagnetic waves relates to their speed of propagation. Mechanical waves travel with a speed that is characteristic of their medium of travel. For example, sound travels much faster in metals than it does in air. Its speed of travel in air is also dependant on the air pressure, temperature, and humidity. In the same way, the speed of propagation of electromagnetic waves depends on the material it is passing through, even though it does not "need" the material for its propagation. This wave travels its fastest in vacuum, with a speed of three hundred million meters per second (300,000,000 m/s or 3x108 m/s) or about 670 million miles per hour. But what is most significant is that this speed, to our knowledge, is the fastest possible way that nature allows for transmission of energy and momentum.
A third feature of electromagnetic waves that separates it from mechanical waves is that the energy that it propagates also depends on its frequency (of oscillation). This feature is related to quantization phenomena that we will study in a future topic. As we will examine later, experimental evidence shows that electromagnetic waves can be represented as if they were made of fixed chunks, or quanta, of traveling oscillations, called photons. These photons all travel with the same speed
photon speed = c = 300,000,000 m/s or 3x108 m/s (in vacuum),
but each individual photon's energy depends on its frequency, n (Greek letter, called nu). To be exact, the photon energy, Eg, is just the product of its frequency value, measured in Hertz, with a constant that is named after the German physicist who came up with this idea, Planck. Planck's constant, denoted by the letter h has a value of 6.63x10-34 Joule.second;
i.e. photon energy in Joules: Eg = h n = (6.63x10-34) (value of photon's frequency in Hz).
The amplitude of the electromagnetic wave, in this photon picture, is a measure of the number of photons that are traveling together to make up the wave. So, again, amplitude relates to the energy of propagation.
Electromagnetic waves, photons, interact with charged entities. Atoms, molecules, and solids all have charges that vary in quality (sign and degree of binding) and quantity among them. As a result, electromagnetic waves of different frequency interact differently with the medium through which they propagate. For purely practical reasons, then, we classify ranges of these frequencies as "bands" and give them specific names. For example, very high frequency photons (those with frequencies larger than 1020 Hz) are called gamma-rays. These photons tend to only interact with the atomic nuclei, which are extremely compact in structure. So, gamma-rays pass through even very thick concrete walls. X-rays are photons belonging to a band of frequencies anywhere from 1016 to 1020 Hz. They can go though material objects too, but they do not have as large a penetration range as the gamma-rays. For example, X-rays of the frequency used in medicine penetrate through most tissue, except the denser bones or tumors. All of these ranges of frequencies together is referred to as the electromagnetic spectrum. Ultraviolet light is invisible to us but is able to give us a sunburn. Strangely, most uv light is blocked by window glass and so if you drive on a hot summer's day with your arm out the car window, it will get sunburned while full sun on you through the car windshield will not give you a sunburn. Visible light is just a very narrow band of this spectrum. Its frequency is of the order of 1014 Hz. Our visual system happens to respond to this frequency of photons by generating electric pulses in the visual nervous system, which is interpreted by our brain as sight, as we will discuss in more detail later. Note that all the colors that we see are in the narrow visible range of frequencies. [An interesting tidbit: the word orange was not introduced into the European language until the 10th century when the fruit arrived from the mid-east; orange did not indicate a color until the 1600's.] Infrared photons and microwave photons have less energy than visible light, but microwaves are able to boil water, while visible light cannot. This has to do with the specific interactions of different types of electromagnetic waves with matter and will be important in our later discussions on choosing the type of laser to use in various applications.
Electromagnetic Spectrum
|
Spectrum of Electromagnetic Radiation |
||||
|
Regions |
Wavelength |
Wavelength |
Frequency |
Energy |
|
Radio |
greater than 109 |
greater than 1 |
less than 3 x 109 |
less than 10-5 |
|
Microwave |
109 - 106 |
0.1 - 0.01 |
3 x 109 - 3 x 1012 |
10-5 - 0.01 |
|
Infrared |
106 - 7000 |
0.0001 - 7 x 10-7 |
3 x 1012 - 4.3 x 1014 |
0.01 - 2 |
|
Visible |
7000 - 4000 |
7 x 10-7 - 4 x 10-7 |
4.3 x 1014 - 7.5 x 1014 |
2 - 3 |
|
Ultraviolet |
4000 - 10 |
4 x 10-7 - 10-9 |
7.5 x 1014 - 3 x 1017 |
3 - 103 |
|
X-Rays |
10 - 0.1 |
10-9 - 10-11 |
3 x 1017 - 3 x 1019 |
103 - 105 |
|
Gamma-Rays |
less than 0.1 |
less than 10-11 |
greater than 3 x 1019 |
greater than 105 |
These values are very approximate and only denote a range. eV is a unit of energy. It is the energy that an electron acquires once it passes through a 1 volt potential difference. It is useful for study of atoms. Note the energy for the visible section.
Questions on Electromagnetic Waves
Last Modified Thursday, 04-Sep-2003



