Nature's Way
Nature does provide working examples of a biological widget maker that successfully bypasses the sticky finger constraint described by Smalley: proteins, genes, organelles, are bioorganic structures that are derived from specific reactions on large molecules carried out with remarkable accuracy and repeatability. Most importantly genes make proteins; they assemble the parts using enzymes, one segment at a time.
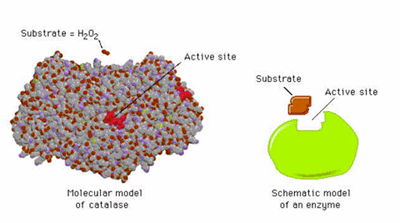
Enzymes are near miraculous catalysts that choreograph biological reactions. Enzymes are Nature’s nanobots. At current levels of understanding of synthetic catalysis, enzymes are ridiculously complicated. Consider the enzyme that attaches the simple molecule hydrogen peroxide, H2O2 to another molecule.
The peroxide is picked up by the large enzymic structure called “catalase”; such attachments are precise as to the tolerated molecular geometry and are part of a group of “shape-selective” catalysts.
As an example of the importance of the shape selectively in the biosciences, the animal disease and the associated 100% fatal human-derived disease called ”mad cow disease” is due to the misfolding of a coil of protein to a flattened sheet that attaches to fatty matter in the brain. The coil itself is a wonderful example of the ability of hydrogen bonding to affect biomolecular architectures. Hydrogen bonding attracts an O atom to the next turn H atom that itself is bonded to a N atom on the outside of the coil. This causes the molecule to locally align and thus coil[7]; normally on the inside of α-helical coils, hydrophobic (i.e., "fat-loving") elements of the protein are protected from the mostly aqueous environment of our bodies. This is a common helical biochemical structure and is essentially entirely due to weak hydrogen bonds.
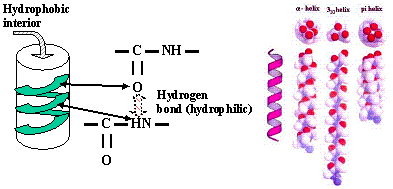
The so-called a-helices described in the previous paragraph can be flattened into two-sided sheets called “beta sheets” when the enzyme that operates on them is defective; this exposes the previously interiorly protected structures to the local environment. This may be the proximate cause of the mad cow disease in humans (“variant Creutzfeldt-Jakob” disease) if these sheets then attach themselves to fatty brain matter.
Another concern of Smalley is the speed possible by nanobots. As we glimpsed above, Nature is sophisticated but slow. Consider:
- Human growth rate 60 kg/20 yrs or approx 0.003 m3/yr.
- Elephant 6,000 kg/20 yrs or approx 0.3 m3/yr.
- Whale 40,000 kg/10 yr or approx 4 m3/yr.
While the rates appear to be increasing with increasing size of each species, they do so mainly because the size of each biological “reactor” is also increasing. The normalized rates should take into account the volumes of each reactor and this works out at 0.05 – 0.1 m3/m3/yr for all of these species.
Except for drugs and perhaps electronic products, most modern materials are needed in millions of tonnes/yr. Assume we wish to make a total of a million tonnes/year[2] of an industrial product with the density of water, some that by volume the product would be 1 million m3/yr. This leads to an estimate of the size of the necessary reactor as 107 m3 - a dauntingly sized piece of equipment with presumably an equally daunting price tag attached.
Can biological nanobots (presumably based on self-assembly using enzymatic controls) produce industrial quantities of materials[3]? Clearly the rates of reaction will have to be much faster than existing biological systems appear to be able to achieve – presumably future nano scientists might try to close the rate gap, but Smalley’s points should be taken seriously.
Footnotes and References
[1]. http://www.cryst.bbk.ac.uk/PPS2/course/section3/helix2.html
[2]. “tonne” is a metric ton or 1,000 kg (or 2205 lb mass).
[3]. The outstanding success in large-scale biological chemistry is fermentation; there has also been some technical, if not much commercial, success in making biodegradable plastics containing natural starch: see, for example, http://www.bact.wisc.edu:81/ScienceEd/discuss/msgReader$10.