Other Condensed Phase Self-Assembly Methods
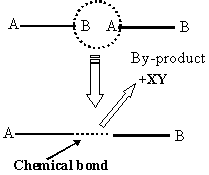
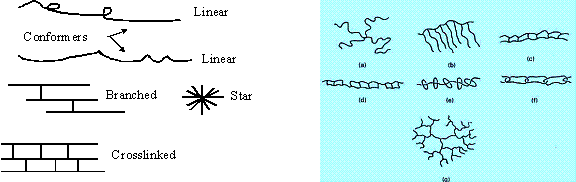
If we stretch the definition of “self-assembly” just a little, the work of many polymer chemists falls under the general title. However, compared to what can be done on a small scale by STM/AFM, & optical tweezers and, on a large scale, by biological systems, the results are comparatively crude. In the simplest scheme, two bifunctional molecules, A-----B can react A to B perhaps by eliminating some simple stable intermediate such as water, an acid, etc. This can be repeated until some other competing reaction occurs or we run out of A-----B monomer. The polymers thus formed may be of differing physical structure called “conformers”. With added functionalities there are many other possible forms the resulting polymers can take as per these inset diagrams.
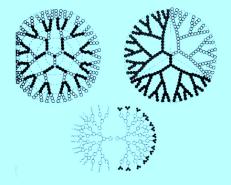
The key point is that chemists can produce these structures by design and, while not yet with the precision that future nanotechnology will expect, some of these various forms are already finding useful applications. For example, the dendrimers shown opposite can be expanded by exposure to aqueous solutions (such as in vivo) and molecules enclosed within their centers can be released into their environment; as such, they are promising candidates to deliver therapeutic drugs[1].
Quantum dots are nano-sized crystals that fluoresce in specific visible colors by virtue of their size when illuminated with a suitable source. The quantum dot is typically a semiconductor and is also a hydrophobe. Their use as biological tagging agents in a hydrophilic environment such as in vivo was not main stream until Dubertret and colleagues[2] were able to attach surfactant-like “phospholipids” to them.
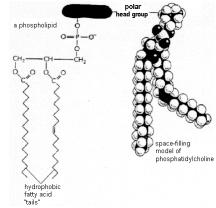
Phospholipids are widely found in biological membranes. The phospholipids are amphipathic (meaning with both hydrophilic and hydrophobic moieties). A lipid molecule has a negatively charged phosphate group and two tails that are hydrophobic hydrocarbon chains - see inset figure[3]. They thus can be attached to the hydrophobic quantum dots and display a hydrophilic outer coating to facilitate in vivo interactions. By carefully controlling the nature of binding agents, treated quantum dots hold the promise of being highly specific and thus to distinguish among biological tissues. The hope is that they will find application in the important fields of imaging and diagnosis. The physical picture of a quantum dot is thus a multi-domain structure with a semiconductor core, perhaps InP, or Cd-Se (although unfortunately Cd is quite toxic), and a dendrimer-like outer coating to compatibilize it with its aqueous-based surroundings. Its external appearance would be similar to the dendrites shown above.
Footnotes and References
[1]. http://pubs.acs.org/cen/coverstory/8034/8034drugdelivery1.html
[2]. B. Dubertret, P. Skourides, D. J. Norris, V. Noireaux, A. H. Brivanlou, A. Libchaber, “In Vivo Imaging of Quantum Dots Encapsulated in Phospholipid Micelles”, 298, # 5599, 1795 (Nov 29, 2002); see also: http://www.sciencemag.org/cgi/content/full/298/5599/1759
[3]. http://img.sparknotes.com/biology/cellstructure/cellmembranes/gifs/phospholipid.gif